Bioequivalence
KAIMRC-BE is a KSA Clinical Research and Bioanalytical company Headquartered at KAIMRC Medical Biotechnology Park with focus on testing generic drug and conducting clinical research.
The objectives of the bioequivalence project are
To establish state of art Bioequivalence facility in KAIMRC/MNG-HA.
To improve the quality of generic drugs and health outcome for KSA and regionally.
To establish strategic presence in the region taking advantage of the market potential.
To build a profitable & cost effective venture.
KAIMRC-BE Business Concept Overview:
- Clinical.
- Bioanalytical.
- Other (TDM, Quality, Abused drugs…..).
Bioanalytical Unit Operation:
- Generic Drugs.
- Therapeutic Drug Monitoring (TDM).
- Quality Control Test for the Pharmaceutical drugs delivered to the MNG-HA and Other Health Organizations and private sector.
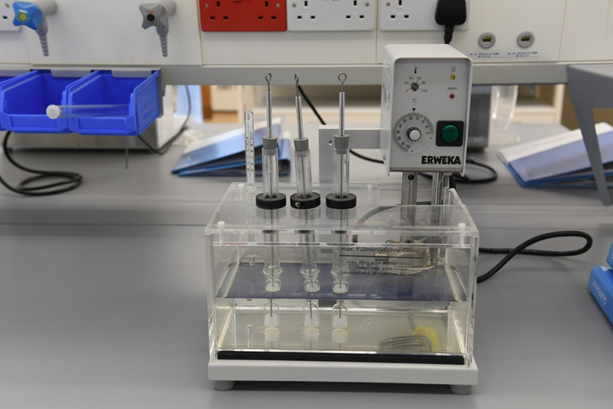
Bioanalytical Lab
Bioanalytical Operations
- 500 m2 in KAIMRC state of art Science park
- High tech instruments such as, LC-MS/MS, UPLC and HPLC , other equipment
- Disintegration Tester
- Dissolution Tester
- Multicheck instrument
- Friability Tester
- Suppository Hardness Tester
- Suppository penetration tester
- Minor Instrument (Balance, pH meter and centrifuge etc..)
- Extraction unit.
- Chemical materials, Buffers, solvents, Standard Reference and other consumables.
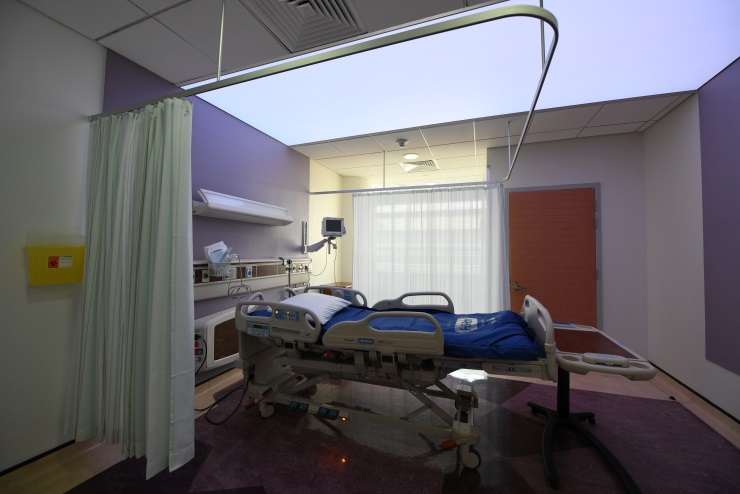
Translational Research Unit
Clinical Unit (Translation Research Unit) Operations
- Volunteer Recruitment.
- Patient Recruitment.
- Drug Administration.
- Initial Focus :
- Generic drug Registration.
- Translational Research.
- Therapeutic Drug Monitoring (TDM).
- Quality Control Test for the Pharmaceutical drugs delivered to MNG-HA and other Health Organizations and pharmaceutical industry.
- Phase-I, Phase-II, Phase III & Phase-IV.
Translational Research Unit Set up:
- Reception
- Ward with 16 Beds
- Phlebotomy Room
- Volunteers Screening Room
- Volunteer Recreation Room
- Centrifuge and Freezer Room
- Chemistry Lab